Interactions between proteins and other molecules are central to the architecture of life, and mapping these interactions is a major bottleneck in developing new therapeutic strategies. The transient and environmentally responsive nature of many disease-relevant protein interactions can make their identification with traditional techniques challenging and resource-intensive. At present, only a small part of the true protein interactome space is susceptible to discovery with existing technology- a problem highly analogous to the demonstrated existence, but unknown composition, of dark matter in the universe. We aim to reveal the nature of this interactomic “dark matter” by inventing photocatalytic proximity labeling technologies explicitly designed for high-throughput discovery of conditional and transient protein interactions.
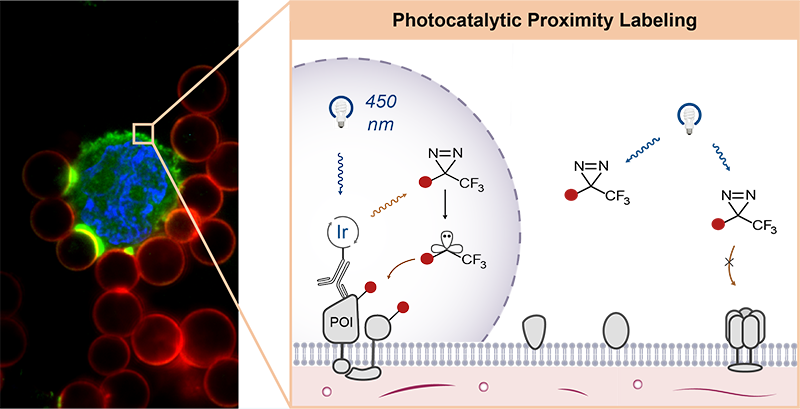
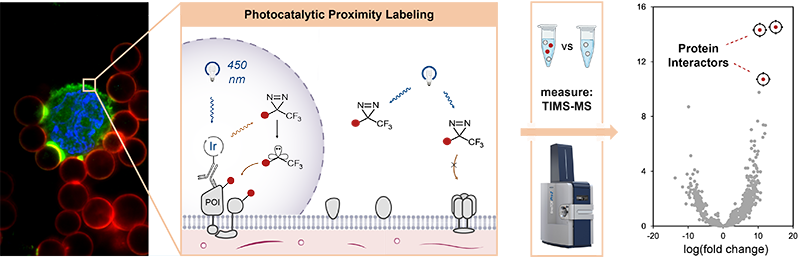
In photocatalytic proximity labeling, a photocatalyst is attached to a targeting modality (antibody, protein, peptide, small molecule) that specifically binds to one protein in a complex biological sample. When it absorbs a photon, it enters an excited triplet state, which can then undergo energy transfer with a nearby “warhead” molecule. The activated warhead can then react irreversibly with nearby proteins, marking them for immunoprecipitation. The sample is then lysed and processed for analysis via IP/mass spectroscopy proteomics. The Geri Lab develops photocatalytic systems for proximity labeling and combines them with cutting-edge mass spectroscopy methods and optical control to discover spatiotemporally specific interactions and to conduct high-throughput interactome surveys.


